COLLAPSING CANS AND BARRELS : DEMONSTRATE THE "POWER OF PRESSURE"

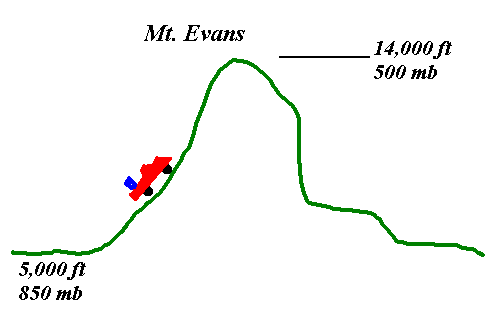
The story goes that Scott Kittelman (or the class TA) took a barrel full of (see below) up Mt. Evans for a party. They drank it all and then closed up the barrel and drove down the mountain. The barrel collapsed because of the increase in air pressure acting on a container with a large surface area.

Physics Lab may have a big one lying around.

Ours is a little one (how could the part crowd finish off the big one (55 gallons) in one sitting):

A MORE ACTIVE DEMONSTRATION:

Questions:
©2000. John Hart, University of Colorado